
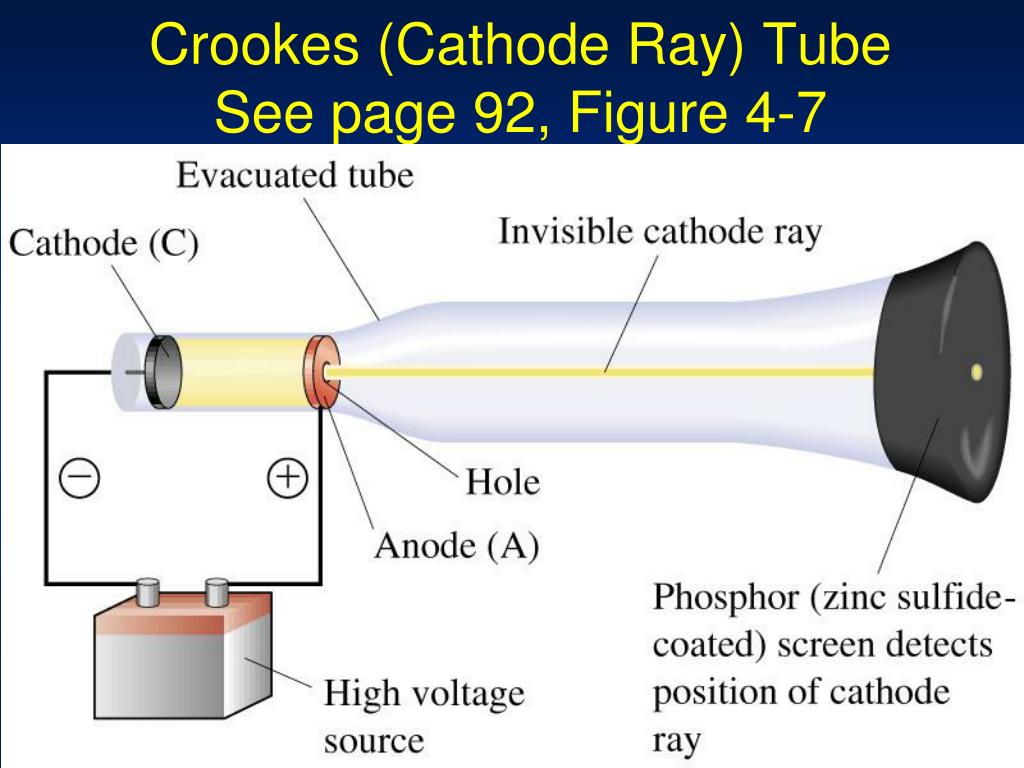
The solenoid that generates the magnetic field has a diameter of 10 cm. One of the phenomenal contributions of Thomson to science is his cathode ray experiment. The simulation below uses metal plates 10 long with a spacing $d=$ 2 cm. The force on an electron due to the electric field is $\vec. They saw a glow from the tube that seemed to emanate f. The electrons were accelerated in the $x$-direction, the electric field was in the $y$-direction and the magnetic field was in the $z$-direction. In the mid 1800s scientists successfully passed an electric current through a vacuum in a glass tube. Some of the electrons pass through a small hole in the plate and form and electron beam that travels to a region where an electric field and a magnetic field was present. A side tube P is used to pump out the enclosed gas so as to obtain the desired low pressure. It is fitted with two metal electrodes named as anode A and cathode C.
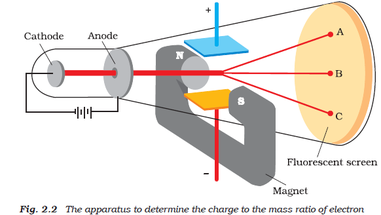
It is a closed tube of length about 30 cm and diameter of about 4 cm. The experiments discussed in this paper were undertaken in the hope of gaining some information as to the nature of the Cathode Rays. J.J.Thomson cathode ray experiment J.J.Thomson used a glass tube called as discharge tube. Describe J.J Thomson’s experiment on the discovery of cathode rays. Thomson (1856-1940) Cathode Rays Philosophical Magazine, 44, 293-316 (1897). The glass tube is attached to a vacuum pump and the pressure inside the tube is reduced to 0.01mm. The electron charge-to-mass ratio was measured by accelerating the electrons through a voltage $V_x$ towards a positively charged plate. A cathode ray tube consists of two thin pieces of metals called electrodes sealed inside a glass tube with sealed ends. He determined that the negatively charged particles (electrons) were much lighter than the positively charged particles. Thomson performed experiments to show that atoms consisted of sub atomic particles that had positive and negative charges. Thomson's experiment to determine the charge-to-mass ratio of electrons


 0 kommentar(er)
0 kommentar(er)
